AI-Driven Solutions Designed to Enhance Compliance,
Optimize Efficiency, and
Connect
Teams
Our AI-driven platforms are built to elevate compliance, sharpen operational focus, and create stronger connections across teams. With intelligent systems that learn and adapt, we turn complex challenges into streamlined, scalable solutions.

AI-Powered Pharmacovigilance for Next-Generation Drug Safety Transforming drug safety with intelligent insights and automation. SafePhV is an advanced AI-driven pharmacovigilance platform designed to streamline drug safety processes, improve accuracy, and ensure compliance with global regulations.

Why Choose SafePhV?
- Comprehensive Database – A robust platform ensuring accurate and reliable safety data.
- AI-Driven Insights – Machine learning capabilities for proactive risk management.
- Regulatory Compliance – Fully aligned with global pharmacovigilance regulations.
- Automation & Workflow Optimization – Reduces manual efforts and enhances efficiency.
- Real-Time Monitoring –Continuous safety surveillance to detect and manage risks.



Pharma Plus is a powerful Enterprise Resource Planning (ERP) software designed to help organizations in pharmaceuticals, biotechnology, healthcare, manufacturing, and distribution manage daily business activities with precision. From business intelligence and financial management to supply chain automation and regulatory compliance, Pharma Plus unifies critical functions into one seamless system.

Why Choose Pharma Plus ?
- Centralized Database – Ensures seamless information sharing across all departments.
- Real-Time Data – Access up-to-date insights for better decision-making.
- Scalability – Designed for both large enterprises and small-to-midsize businesses.
- Regulatory Compliance – Includes integration with UK HMRC VAT submissions for secure, automated tax reporting.
- Demand Planning System (DPS) –Automates supply chain management for optimized inventory and production.

Scan Plus is an innovative serialisation and compliance platform designed to protect the pharmaceutical supply chain from counterfeit medicines. Built for pharmaceutical manufacturers and contract manufacturing organisations (CMOs), Scan Plus ensures regulatory compliance while streamlining serialisation management and data handling.
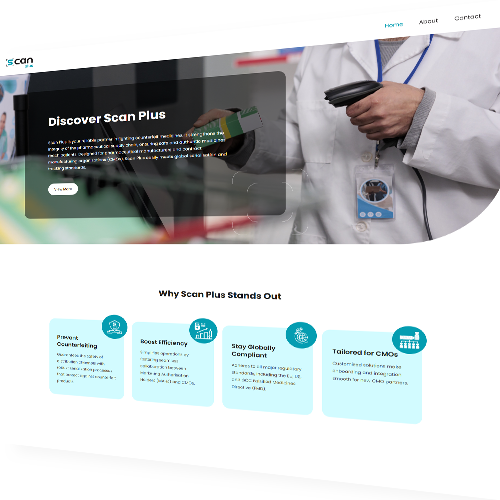
Why Choose Scan Plus ?
- Prevents Counterfeiting – Strengthens distribution channels with robust serialisation processes.
- Boosts Efficiency – Facilitates seamless collaboration between Marketing Authorisation Holders (MAHs) and CMOs.
- Ensures Global Compliance – Fully adheres to EU, US, and GCC Falsified Medicines Directive (FMD) regulations.
- Tailored for CMOs – Customised solutions ensure smooth onboarding and integration.



QMS Plus is a powerful and intuitive Quality Management System (QMS) designed to help pharmaceutical and life sciences companies maintain compliance, improve efficiency, and streamline critical quality processes. From document control and audits to training and change management, QMS Plus provides a centralized, fully integrated platform that ensures regulatory alignment and operational excellence.

Built to adapt to businesses of all sizes, QMS Plus combines automation, analytics, and regulatory expertise to simplify compliance with global standards such as GAMP 5, EU Annex 11, and 21 CFR Part 11. Whether you're a startup or a global enterprise, QMS Plus offers a scalable, flexible solution that integrates seamlessly into existing workflows.

PMS Plus is a cloud-based project management platform designed to help life sciences organizations streamline workflows, optimize resources, and track progress with real-time data. It empowers teams to plan, execute, and deliver projects efficiently.

Why Choose PMS Plus ?
Built for the complexities of R&D and product development, PMS Plus enhances project visibility, automates key processes, and ensures teams stay aligned with business goals.



Connect Plus is revolutionizing global pharmaceutical and MedTech collaborations, offering an innovative space where companies can explore opportunities, raise enquiries, and stay ahead in the evolving healthcare landscape. With a focus on industry expertise international reach, and cutting-edge innovation, Connect Plus empowers organizations to connect, grow, and lead in their respective fields.

Why Choose Connect Plus?
- Expanded Business Opportunities – Build valuable partnerships and discover new markets.
- Streamlined Communication – Engage effortlessly with industry leaders and key decision-makers.
- Innovation-Driven – Stay ahead with the latest pharmaceutical and MedTech advancements.
- Global Reach – Connect with partners worldwide to maximize growth potential.
- Expert Guidance –Receive tailored support from our experienced business development team.

e-CTD Plus streamlines the creation, management, and submission of electronic Common Technical Documents (eCTD), ensuring compliance with global regulatory standards. Designed for pharmaceutical and life sciences organizations, it accelerates approvals and simplifies dossier preparation.
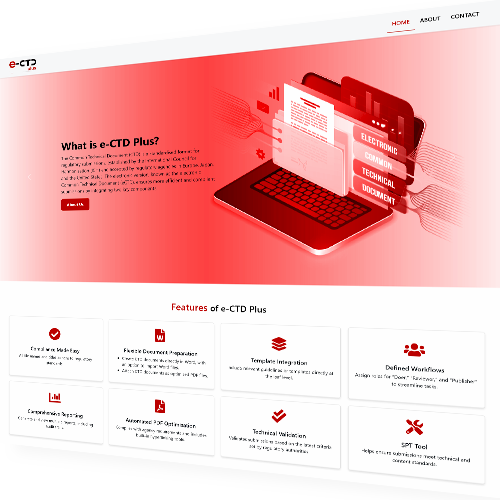
Why Choose e-CTD Plus ?
Built to meet ICH and regional requirements, e-CTD Plus eliminates the complexity of regulatory submissions. It enhances document accessibility, ensures compliance, and optimizes workflows for faster approvals.



Recruit Plus is an advanced recruitment and onboarding platform designed to streamline hiring processes. It automates candidate management from application submission to onboarding, enhancing efficiency and collaboration among HR teams, recruiters, and hiring managers.

Why Choose Recruit Plus ?
With AI-powered automation and seamless integration, Recruit Plus eliminates manual inefficiencies, ensuring a structured, compliant, and data-driven recruitment process. It enhances collaboration, reduces hiring time, and improves the overall candidate experience.